The JBI's Alumina Process Pilot Plant
The pilot plant was constructed in 1984 to upgrade the scientific and technological capabilities of the Jamaica Bauxite Institute (JBI). Its construction was funded jointly by the Government of Jamaica and the United Nations Financing System for Science and Technology
The pilot plant was designed with a high degree of flexibility of operation to make it capable of:
1
2
3
4
The plant is a complete closed circuit Bayer process facilityembracing the unit operations of an industrial scale Bayer alumina plant up to the hydrate production stage. It can operate at digestion temperatures up to 2600C and can process a minimum of 20 tons of bauxite continuously over a one-month period. The minimum production rate is 10 kg per hour of alumina. Bench scale simulations can be carried out in our Laboratories on a number of parameters, prior to the pilot plant study stage. The plant is equipped with online slurry density gauges, magnetic flowmeters, conductivity meters for the measurement of a/c ratios, temperature and pressure recording instruments.
The pilot plant has the capability of processing all types of bauxite, from e.g. Australia, Brazil, Jamaica, etc.
The small scale of unit operations allows for close control and monitoring of processes and the economical testing of all types of blends of bauxites, flocculants, dispersants, coagulants and liquor purification agents as well as equipment including process instruments, agitators, thickeners, pumps, valves and heat exchangers. Steady state conditions are achieved in a short time, and results of tests can be quickly obtained. Process parameters can be varied rapidly and at will.
The plant utilizes a single stream process, which is more energy efficient than the two stream process. The plant has an adequate surge capacity for the movement of fluids from one unit operation to another.
Projects for which the pilot plant was utilised include developmental studies on chemicals used in Bayer Process plant operations worldwide and impurities removal systems.


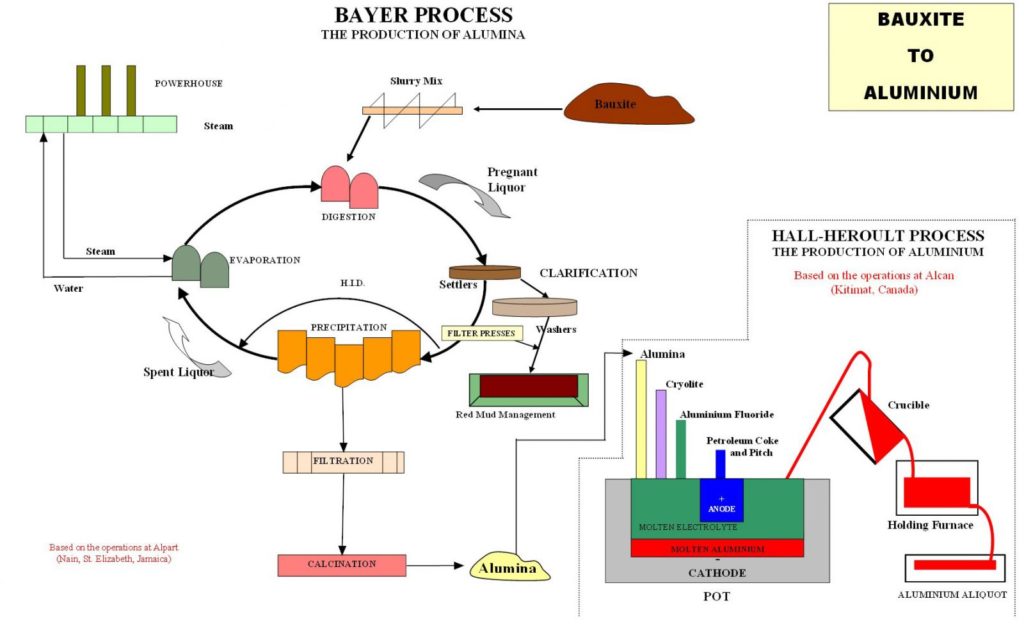
The Bayer Process
The production of alumina from bauxite is carried out by the use of the Bayer Process. The process was invented in 1888 by an Austrian, Karl Joseph Bayer.
Bayer discovered that when bauxite was mixed with caustic soda, the alumina content in the bauxite would dissolve and separate itself from the other components, such as iron and silica, which would remain in solid state.

The JBI's Alumina Process Pilot Plant
Bauxite is the major ore for aluminum production. Alumina is extracted from bauxite by the Bayer process. The waste slurry generated in this process is called red mud and its solid fraction is called bauxite residue. Bauxite residue contains several interesting minor or trace elements, among which are the rare-earth elements (REEs) as well as scandium (Sc) and Yttrium (Y).
These Trace Elements are characterized at the ppm level, i.e. 1 milligram per 1 kilogram (mg/kg), and a pilot-plant study performed by Japanese Scientist at the Jamaica Bauxite Institute (JBI) has shown that the REE can be successfully extracted. In Red-Mud the total REEs composition is known to be approximately ~ 900ppm.
More than 90% of the trace metal value in Red-mud can be attributed to the presence of one of these REEs, namely Scandium (Binnemans et al., 2013). This significance has been recognized by the leading researchers and as such research is underway as to secure the most economically feasible way to extract and concentrate the scandium recovered from the Red-Mud; one such research is now underway here in Jamaica at the Jamaica Bauxite Institute (JBI).
|
Trace Elements
|
Elemental State
|
Expressed as Oxides
|
Uses
|
|
|---|---|---|---|---|
|
Scandium
|
Sc
|
Sc2O3
|
Super alloy; Sputtering target; Nuclear; Aero alloy
|
|
|
Lanthanum
|
La
|
La2O3
|
Ceramics; Electronic; Crystals; Phosphors
|
|
|
Cerium
|
Ce
|
CeO2
|
Catalyst, glass, phosphors and polishing powders.
|
|
|
Praseodymium
|
Pr
|
Pr6O11
|
Pigment; Ceramic glaze
|
|
|
Neodymium
|
Nd
|
Nd2O3
|
Glass; Ceramics; Alloy; Laser crystal; Electric
|
|
|
Samarium
|
Sm
|
Sm2O3
|
Catalysts; Ceramics; Glass; Neutron absorption
|
|
|
Europium
|
Eu
|
Eu2O3
|
Phosphors for lamp, color TV, X-ray and other luminescent materials; Glass; Crystal
|
|
|
Gadolinium
|
Gd
|
Gd2O3
|
Phosphor; Neutron absorption; Optical glass; Electronic; GGG materials; Crystals; Ceramics
|
|
|
Terbium
|
Tb
|
Tb4O7
|
Phosphor; Optical dopant; Electronic; Ceramics
|
|
|
Dysprosium
|
Dy
|
Dy2O7
|
Halide lamps; Optical fiber; Crystal dopant; Ceramics; Phosphors
|
|
|
Holmium
|
Ho
|
Ho2O3
|
Halide lamps; Optical fiber; Crystal dopant; Ceramics; Phosphors
|
|
|
Erbium
|
Er
|
Er2O3
|
Halide lamps; Optical fiber; Crystal dopant; Ceramics; Phosphors
|
|
|
Thulium
|
Tm
|
Tm2O3
|
Phosphor; Optical dopant; Electronic; Ceramics
|
|
|
Ytterbium
|
Yb
|
Yb2O3
|
Phosphor; Optical dopant; Electronic; Ceramics
|
|
|
Lutetium
|
Lu
|
Lu2O3
|
Laser crystal; Optical fiber; Optical dopant; Ceramics; Phosphors
|
|
|
Promethium
|
Pm
|
PM203
|
Phosphor; X-rays
|
Phosphor; X-rays
|
|
Yttrium
|
Y
|
Y203
|
additive to alloys, lasers, microwave filters for radars, catalyst
|